What is Fill-Finish?
Learn more about the final stage of the bioprocess, fill-finish, where the product is packaged in its final, sterilized container.
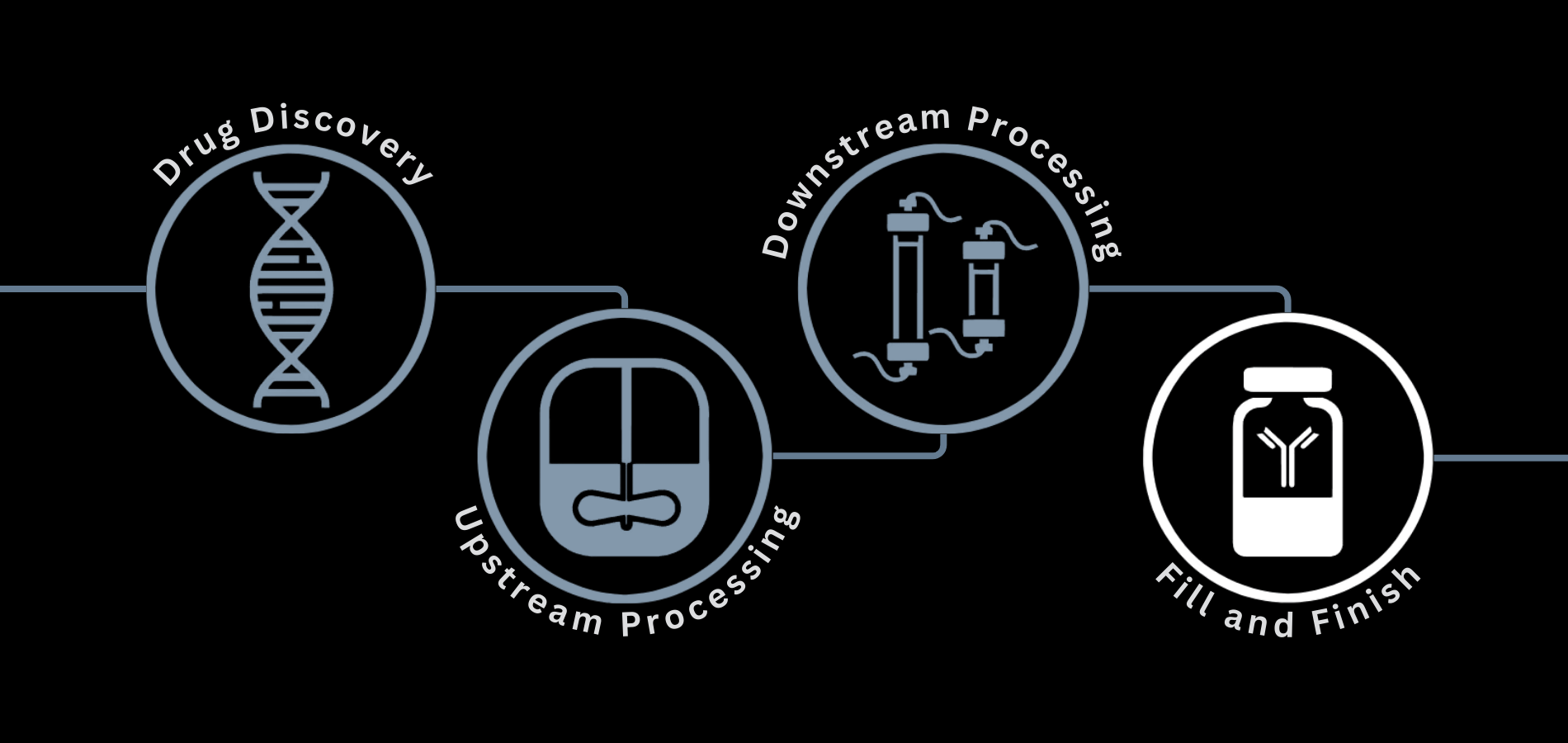
What is Fill-Finish in Biopharma?
Fill-finish comes at the end of the biopharmaceutical manufacturing process. Filling refers to adding the product into a container such as a vial, cartridge, or syringe. Finish refers to final sterilization, standardization, and methods used for filling. It is important that fill-finish occurs in a controlled, sterile environment, with the correct methods for maintaining sterility and other industry standards.
Fill-Finish and the COVID-19 Pandemic
Fill-finish has become an increasingly hot topic in the biopharma and bioprocessing industries following the COVID-19 pandemic. Due to the high volume of vaccines needed, many countries, including the U.S. dealt with a variety of fill-finish bottlenecks. This brought the need for high-volume fill-finish equipment to the attention of many major companies.
Steps to Fill-Finish in Biopharma
The steps of the fill-finish process can greatly differ depending on the product being packaged and the final container. However, fill-finish can generally be broken down into the following steps: container preparation, product sterilization, filling, capping & sealing, inspection & labeling, and packaging & storage.
Container Preparation
The first step is choosing the appropriate container for the final product, and preparing as needed before the filling process even begins. Preparation can include washing, arranging, cleaning, and sterilizing containers.
Product Sterilization
After the upstream and downstream bioprocesses, you have your final product. However, before it can be packaged, it needs to undergo any final filtering and sterilization that would make it safe for its intended use.
Filling
Filling, the next step, must be completed in a controlled and sterile environment. Once the proper environment is established, the drug product can start to be fed into the automated filling machine that was chosen for the process. Once the sterile containers are filled with the product, stoppers are put in place as a preliminary seal, keeping the inside of the container clean and sterile.
Capping & Sealing
After the temporary stopper is put in place, they can be replaced with the final cap, which are typically crimped to ensure that a completely sealed closure is created. Many final-fill containers can be capped by an automated machine. The cap that is fixed to the container at this stage will be the final product.
Inspection & Labeling
A critical part of the filling process, all filled containers must be inspected once complete, which can be done manually, semi-automatically, or fully automatically. The purpose of the inspection is to find any defects. This can include particulates, cosmetic issues, or container closure defects. Once the container and product passes inspection, labels are affixed to the container with all of the product information gleaned through the drug discovery process.
Packaging & Storage
At this point, the fill-finish process is essentially complete, and the completed product needs to be packaged and stored so that they are ready for distribution. Many times, packaging will involve a vaccine or medication trays. All containers are stored until it is time to ship to retailers, healthcare providers, or other external locations.
Bioprocessing Equipment for Fill-Finish
High Purity New England, a Getinge company (HPNE) is a leading manufacturer and distributor of bioprocessing equipment, and has a range of solutions ideal for the fill-finish process.
HPConnexx™ Single-Use Assemblies are the flagship product at HPNE. With brand agnostic designs, an expert team, and an extensive material compatibility resource library, HPNE is equipped to create the ideal assembly for your fill-finish process. All assemblies are manufactured in their ISO 7 Class Cleanrooms, and are shipped in transparent, puncture-resistant bags.
They also carry a range of single-use and multi-use FlowMaxx Pro Quaternary Diaphragm Pumps. There are six scalable sizes available, with flow rates from 1mL to 360L. FlowMaxx Pro integrates both pressure and flow sensors, and provides continuous, real-time information. For ease-of-use, the pump includes a quick start up, enlarged HMI screen.
Additionally, they carry Single-Use Precision Pumps from Quantex, which can provide accurate dosing over a range of flow rates, temperatures, and viscosities. It allows you to reduce damage to cells due to the gentle nature of the pump.
As a proud Getinge company, HPNE also supports Getinge's DPTE_BetaBag® and Sterile Isolator Technology, both ideal for your final-finish process.
Share article
About HPNE
As the industry needs grow, High Purity New England, Inc. continues to supply the biopharmaceutical industry with a range of innovative products, from drug discovery and development to fill-finish, including their flagship product, custom single-use assemblies, as well as pumps, sensors, bioreactor systems, storage and handling solutions and other single-use solutions. Along with their own manufactured products for the global market, they are also a distributor for more than 18 brands in North America.