What is Drug Discovery?
Often taking more than a decade, learn more about all the steps that are required for successful drug discovery resulting in a new pharmaceutical product on the market.
What is Drug Discovery?
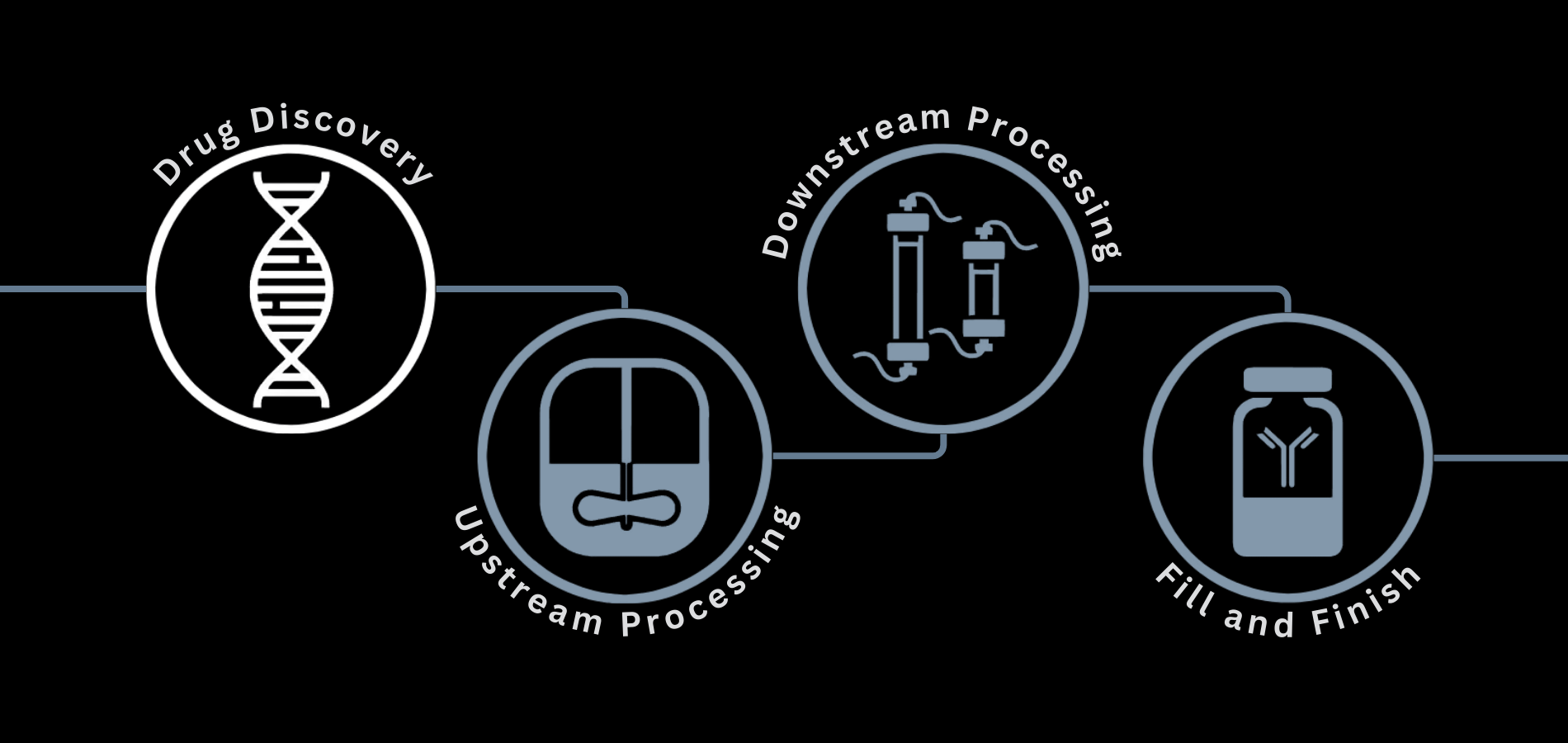
Drug discovery is a long, multi-phase process that can take over a decade, with the ultimate goal of creating a new pharmaceutical product and getting it approved for sale on the market.
Due to the length of time it takes to create a new product, an individual may be part of only 3-4 new product discoveries from start to finish in the length of their career. Additionally, when distributing the product internationally, drug discovery may include verifying that the drug meets multiple varying safety, quality, and effectiveness standards.
Steps for Drug Discovery
The drug discovery process can be broken down into five main steps: discovery & development, preclinical research, clinical development, market launch & regulatory approval, and post-market monitoring.
Discovery & Development
The first step, discovery & development has the goal of identifying the target, and validating that it will be effective for the determined cause. When beginning research, there may be thousands of potential compounds for the medical treatment you are seeking to create. In this stage, it is also critical to thoroughly understand the disease you are attempting to treat, including underlying factors, cellular structures, and more.
Research for identifying the target typically occurs in a lab space. Any identified possible targets must be capable of meeting any possible future commercial and clinical requirements, and must be a safe and efficacious solution for the indicated ailment you are trying to treat. In the discovery & development stage, targets are narrowed down so leading compounds may be tested in the next step, pre-clinical research.
Pre-Clinical Research
In this step, target candidates from the previous stage go through a variety of tests to ensure they will be safe for testing in humans during clinical development. These studies are typically fairly small, and can feature lab tests, as well as animal testing. Once preclinical testing is complete, the research on the tested compound must be presented to and approved by the regulatory authority where the drug is in development or intended to be distributed.
In the United States, research results are presented to the Food and Drug Administration (FDA), who require testing be done using good laboratory practices (GLP). Ultimately, the FDA is looking for proof and details regarding toxicity levels and dosing levels.
Clinical Development
Clinical development is commonly known in phases: phase I, phase II, and phase III. Throughout these phases, the size and scope of testing increases. It is not uncommon for organizations to release their progress publicly at this stage. At this stage in the drug development process, drugs have been proven safe, and are tested on humans to test effectiveness.
During phase I, around 20-80 individuals in relatively good health will volunteer to test the candidate to ensure it remains as safe as the pre-clinical trials indicated. Then, in phase II, the testing population is raised to around 100-300 individuals. For this phase, individuals with the ailment being treated are selected to participate. Taking up to two years, this is the stage where researchers start to perfect dosing minimums and maximums.
In the final phase, phase III, researchers are looking to find any possible side effects using a wide range of one thousand or more patients. Any side effects that are discovered during this phase will be listed on the final packaging of the completed drug.
This stage also involves comparing the newly developed treatment to any previously discovered, standard treatments that are already on the market. Finally, this stage will collect additional data on safety and efficacy, which was started in phase I and II.
Market Launch & Regulatory Approval
Once a drug candidate has successfully passed all three clinical trial phases, it is then reviewed by the necessary regulatory body. An application will be submitted by the researching company, and the regulatory body must ensure that the data collected during clinical and preclinical trials proves that the drug candidate will be effective in treating the determined disease or ailment, and will be safe to use.
In the United States, a New Drug Application (NDA) must be submitted to the FDA for market and regulatory approval. Once approved, the finished drug product may enter the market for the population studied.
Post-Market Monitoring
Even though the finished drug has been thoroughly tested, regulatory bodies continue to monitor possible health and safety concerns of drug products that have entered the market. Consumers are able to report complications they experience as a direct result of interaction with the drug product. In rare cases, a regulatory organization may require the drug developer to conduct an additional phase IV study.
Bioprocessing Equipment for Drug Discovery
High Purity New England (HPNE), a leading manufacturer and distributor of bioprocessing equipment, has a variety of solutions that are useful throughout the drug discovery process.
When testing under the hood, it may be necessary for you to be working with sterile components. Eliminate the aggravation of contaminating your entire component lot when removing a single-part with HPNE’s Sterile Individually Packaged Components. With each part individually double-bagged, you can remove one component while the rest remain sterile and ready-to-use when you need them.
Additionally, for any step of your process, HPNE offers off-the-shelf and fully customizable HPConnexx Single-Use Assemblies. Promising fast lead times, no minimum order quantities, and a brand agnostic design process, HPConnexx sets itself apart from the rest. Their expert team has decades of combined experience, allowing them to develop the perfect assembly for your specific needs every time.
For real-time insights into flow and pressure, High Purity New England offers a range of FlowMaxx Pro Quaternary Diaphragm Pumps. Scalable across six sizes with flow rates ranging from 1mL to 360L, FlowMaxx is the perfect solution for a variety of steps throughout the drug discovery process.
Finally, HPNE offers a sustainable alternative for Adherent Cell Expansion, the CellScrew® from Green Elephant Biotech. Its innovative design incorporating Archemedian Screws, a central tube, and concentric cylinders creates a large cell culture area without taking up much space.
Share article
About HPNE
As the industry needs grow, High Purity New England, Inc. continues to supply the biopharmaceutical industry with a range of innovative products, from drug discovery and development to fill-finish, including their flagship product, custom single-use assemblies, as well as pumps, sensors, bioreactor systems, storage and handling solutions and other single-use solutions. Along with their own manufactured products for the global market, they are also a distributor for more than 18 brands in North America.